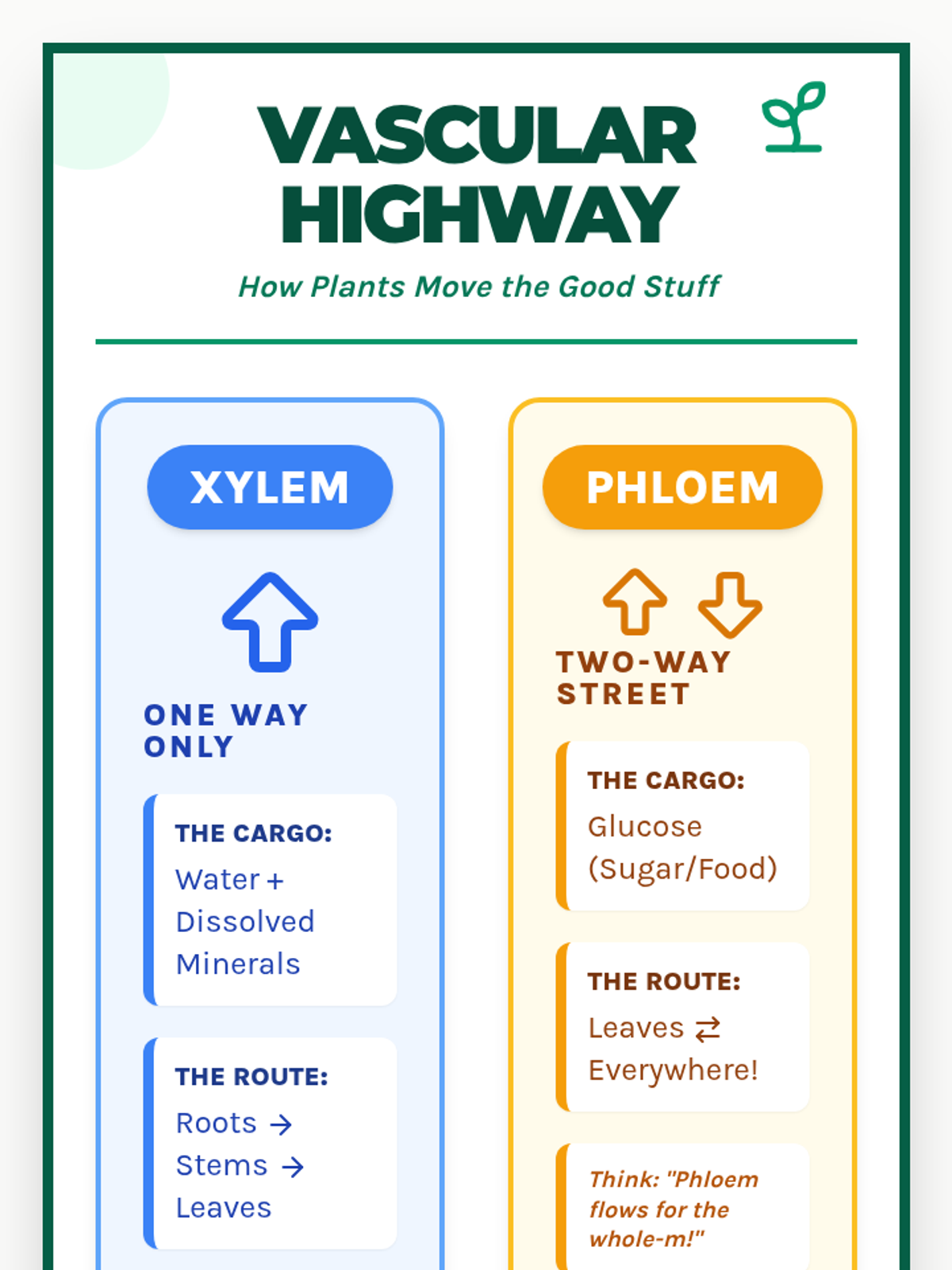
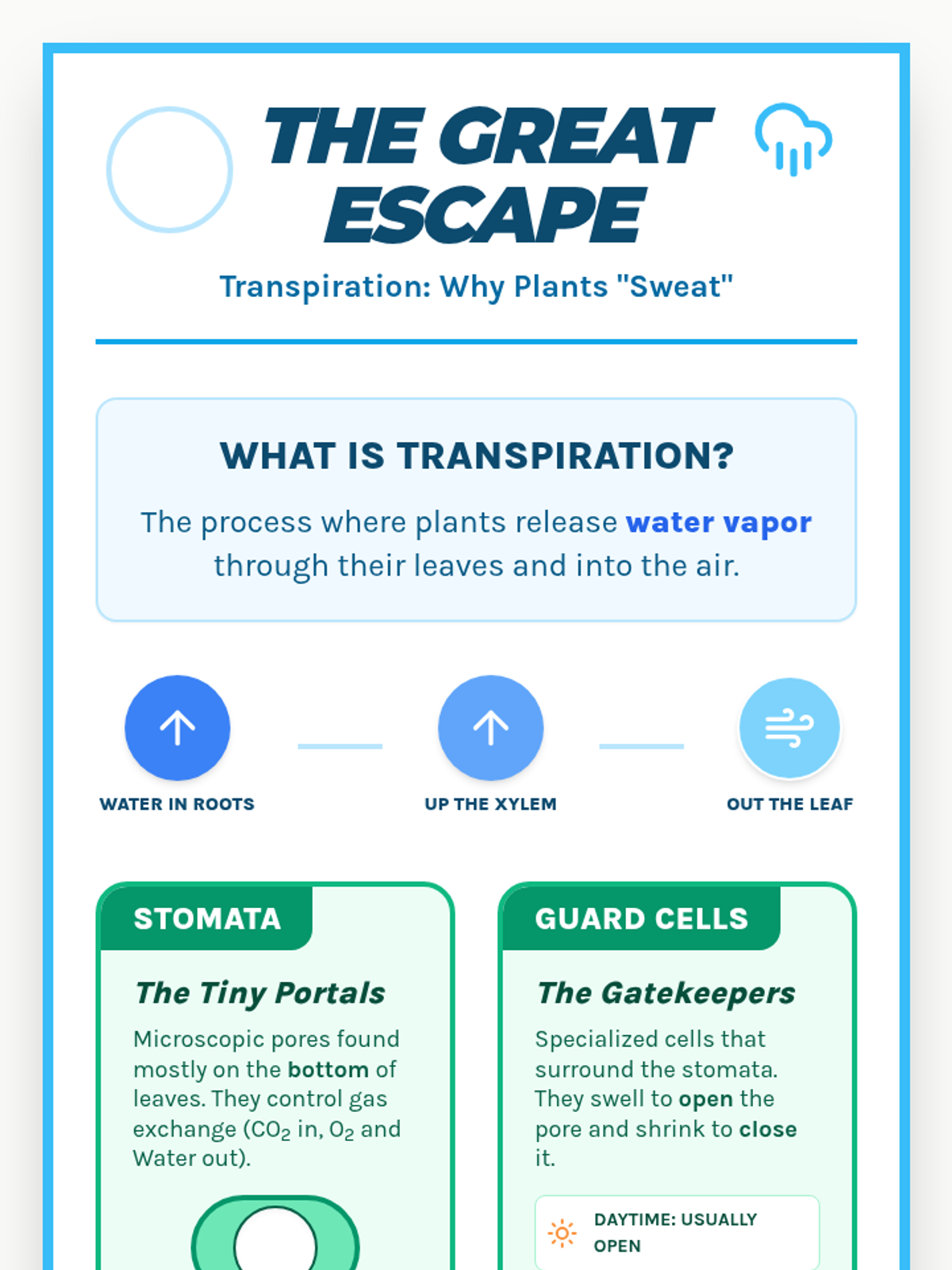
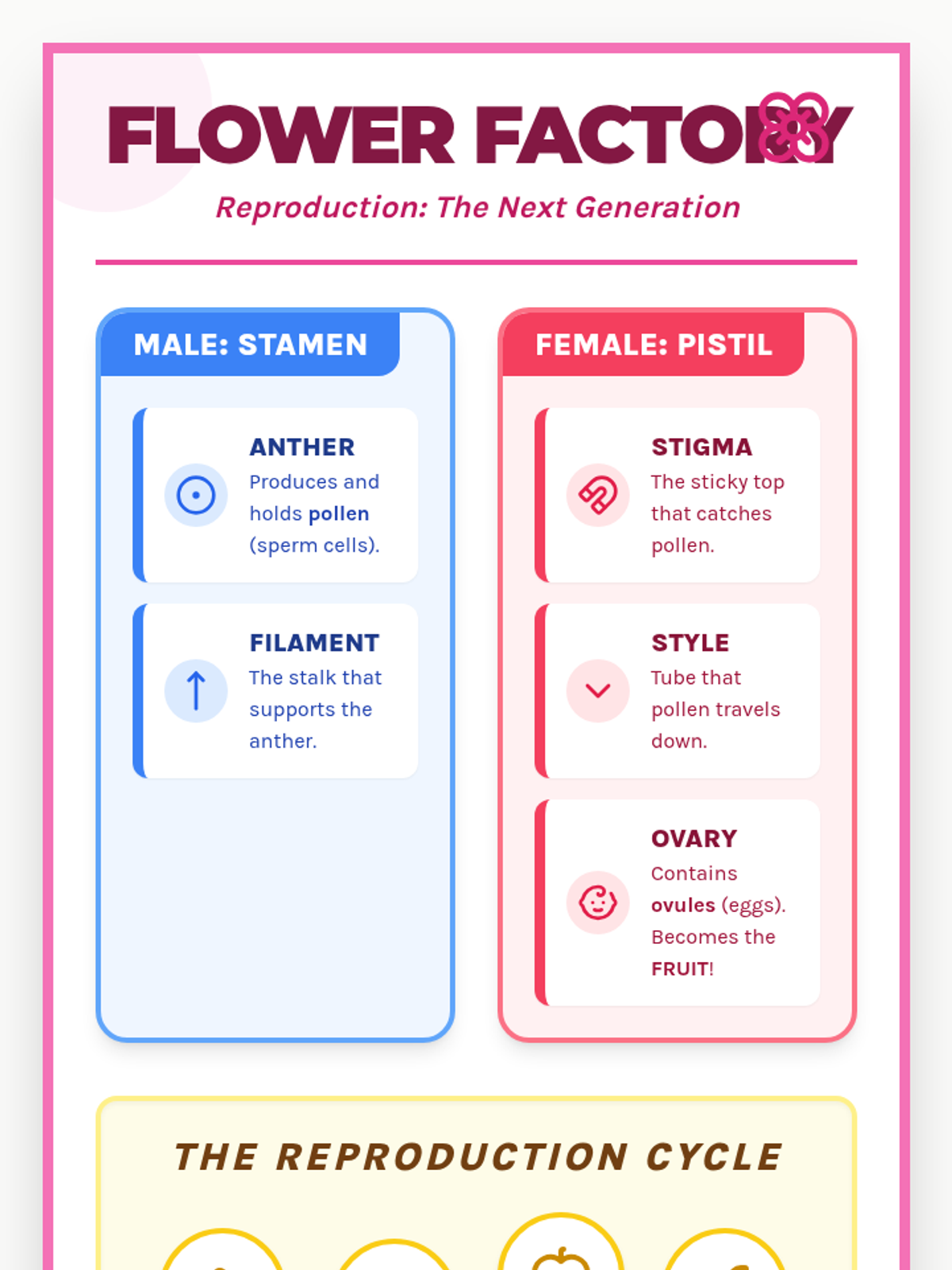
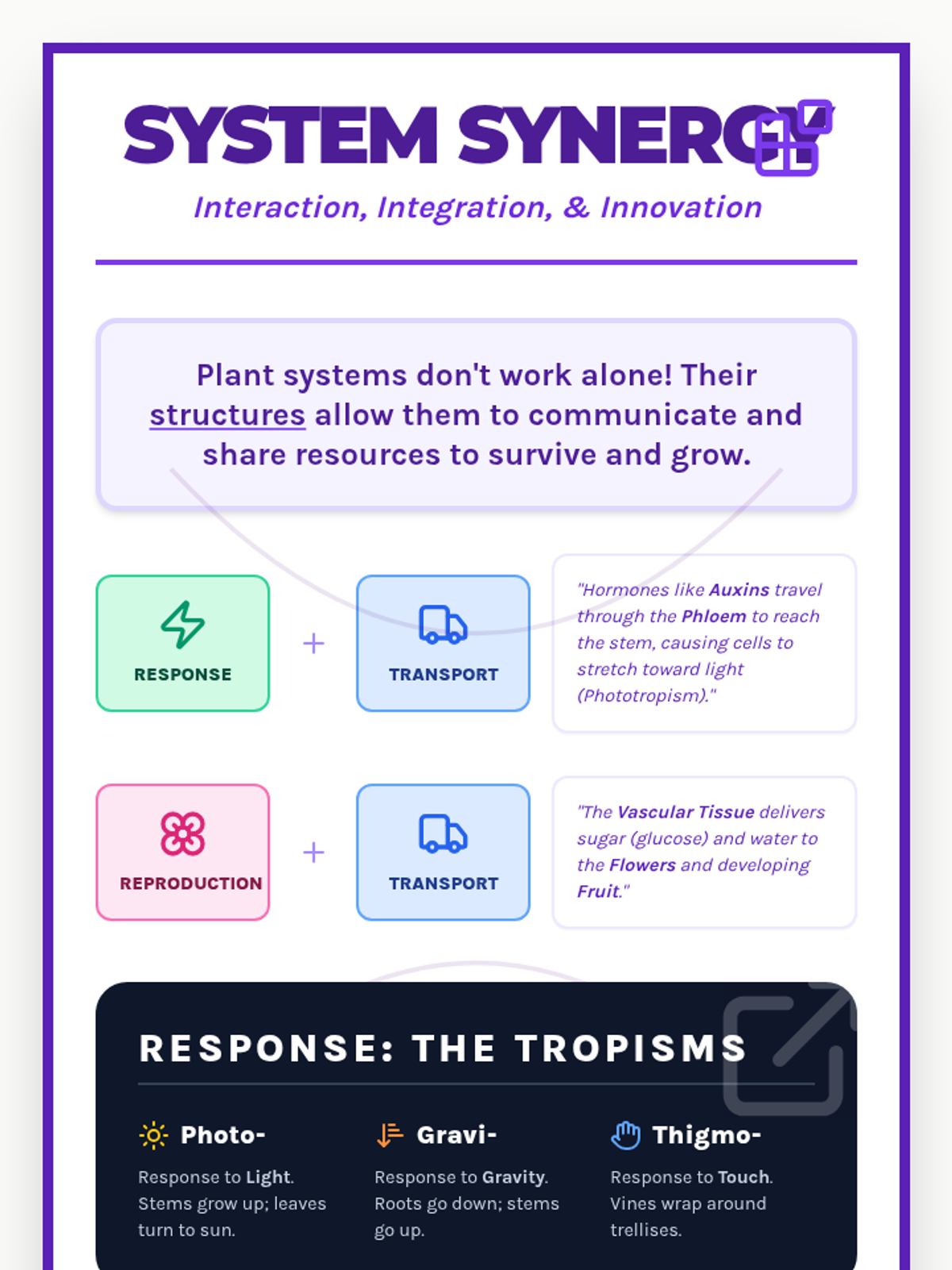
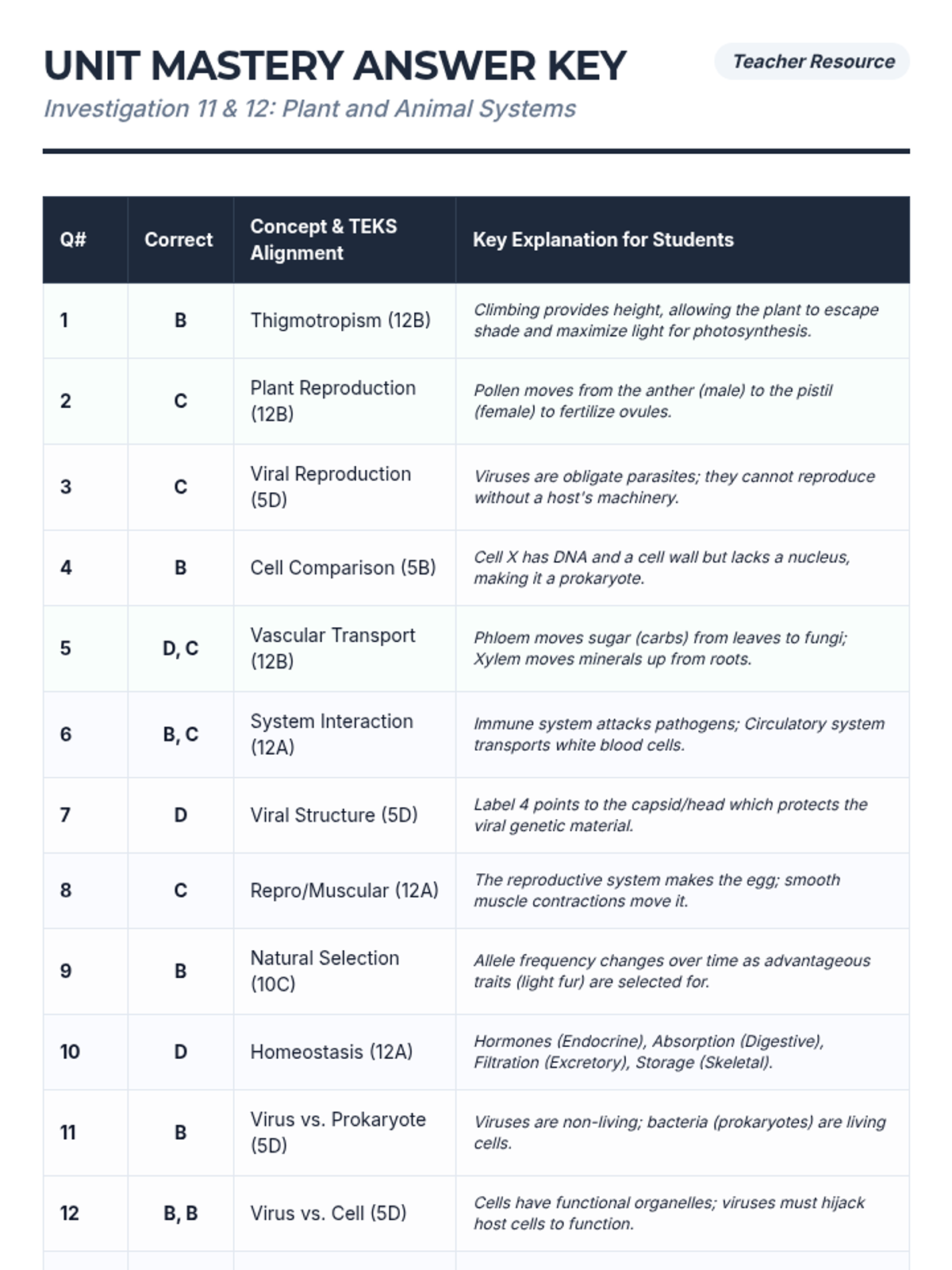
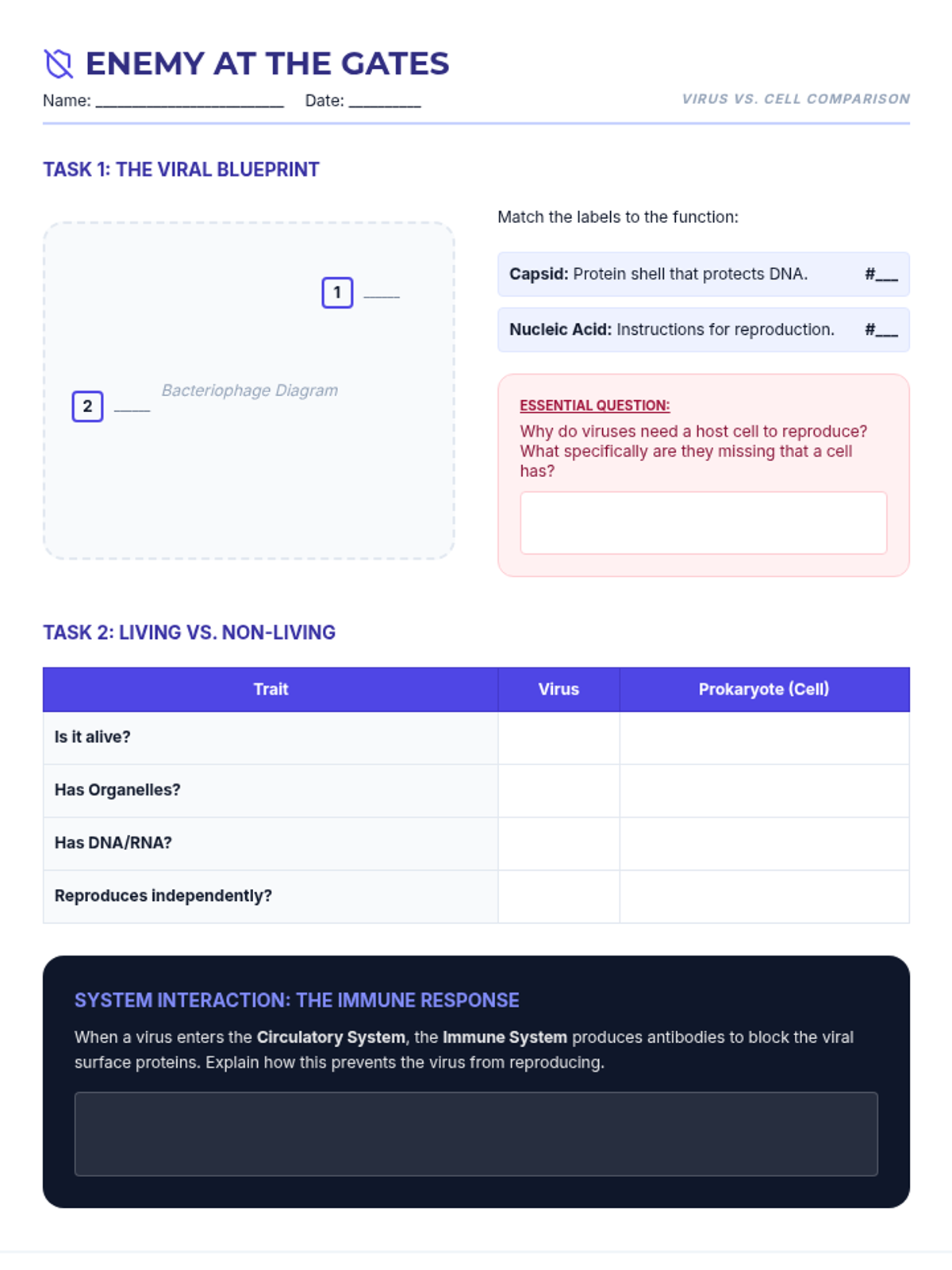
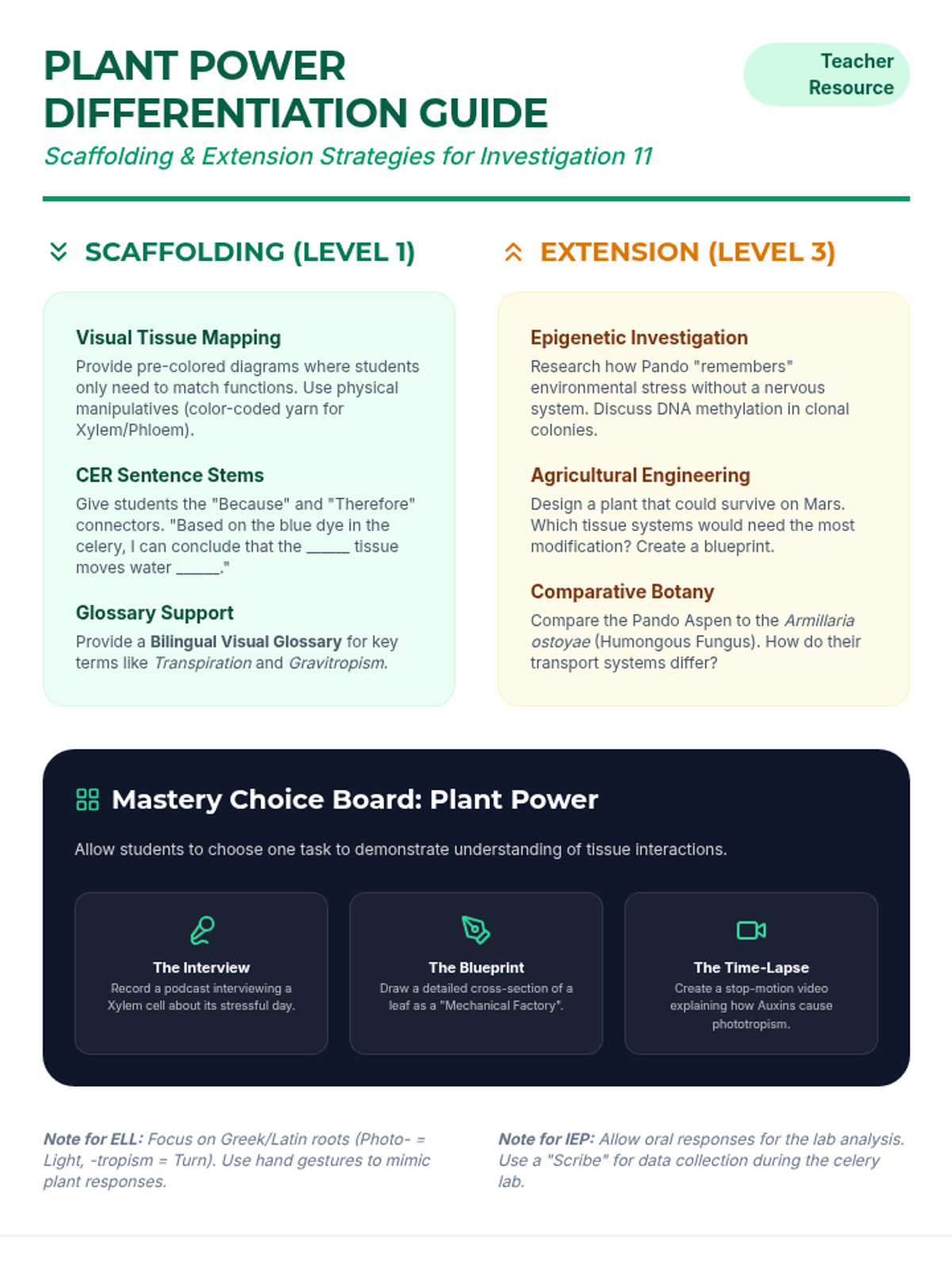
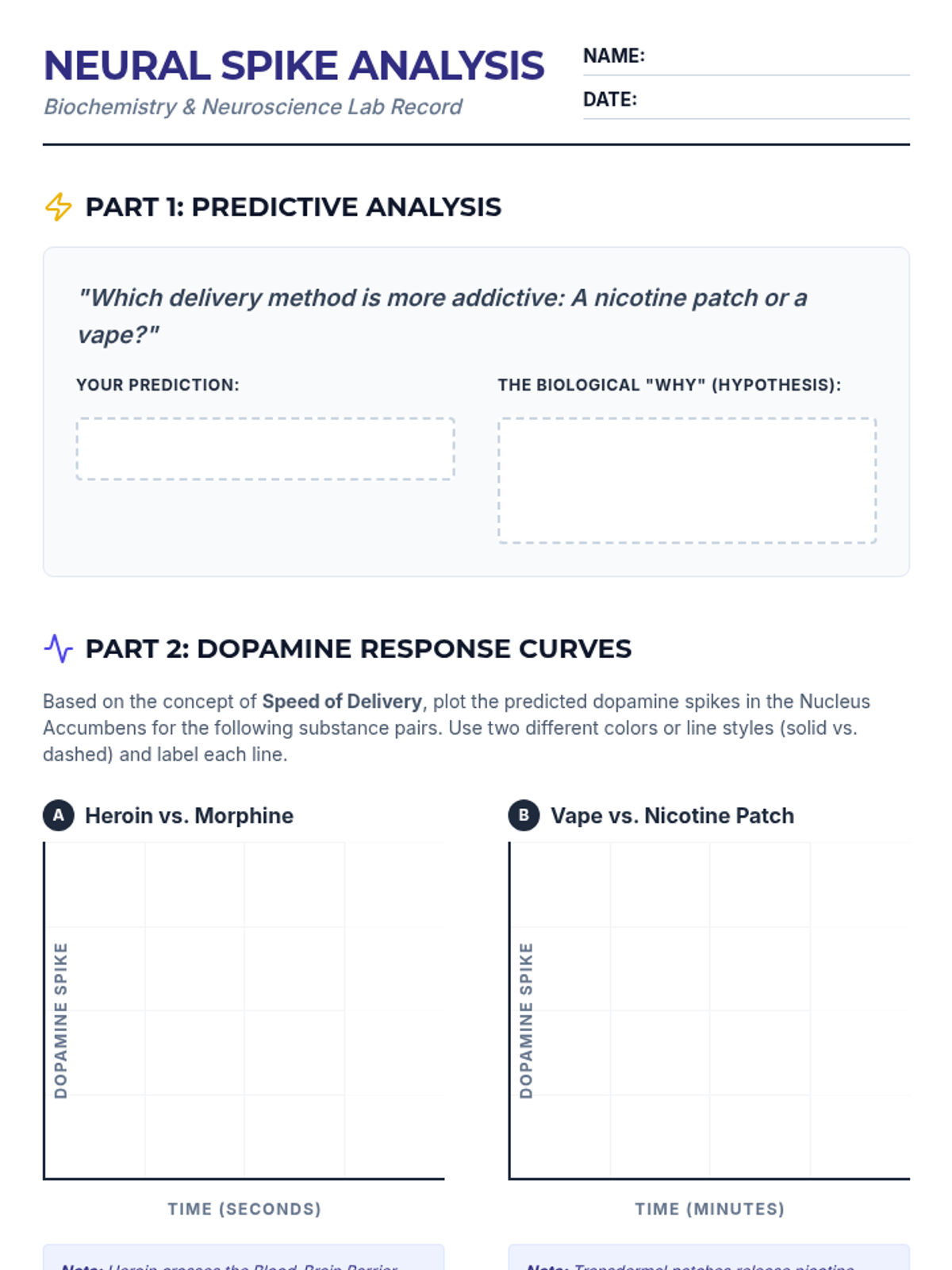
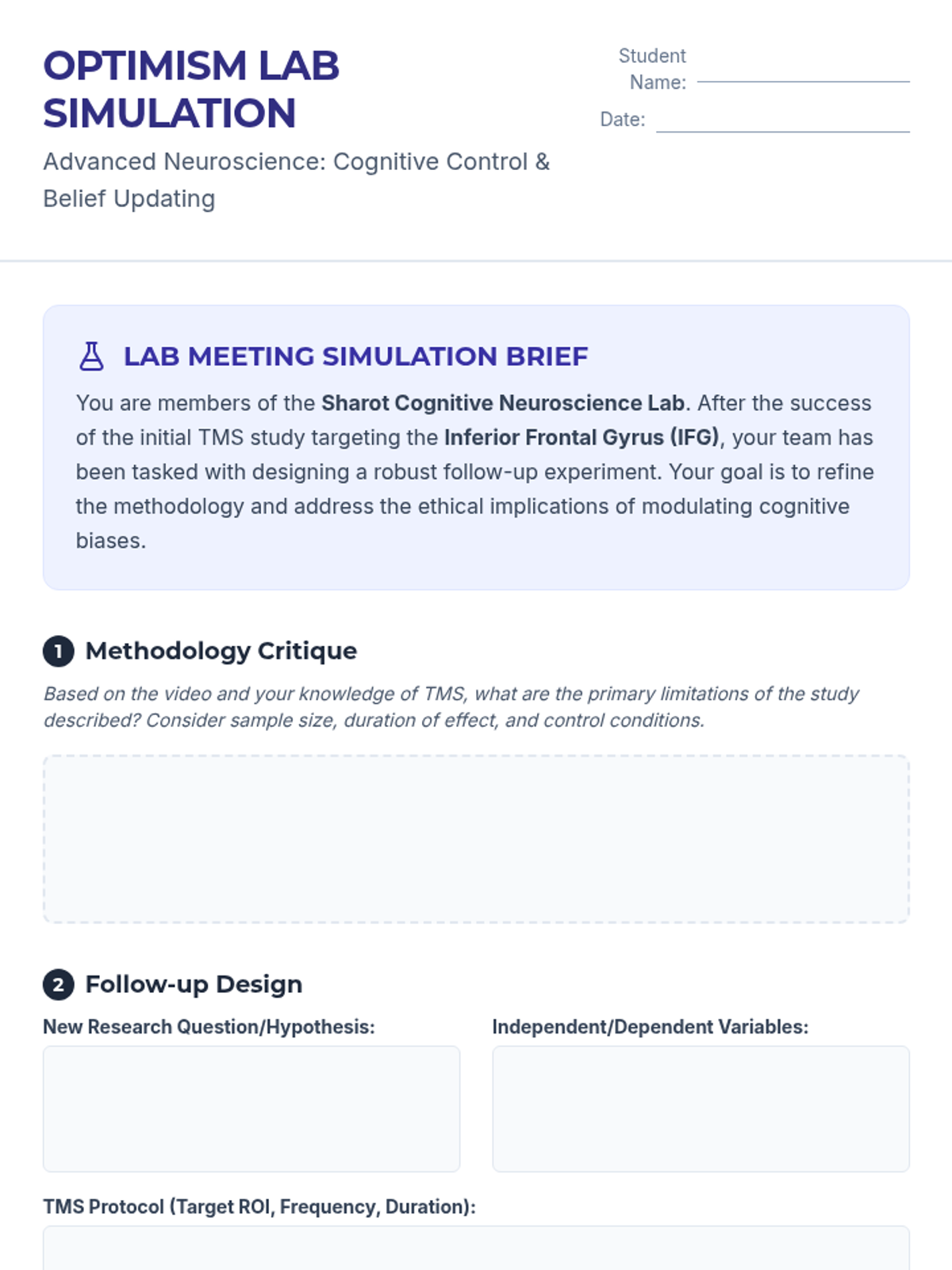
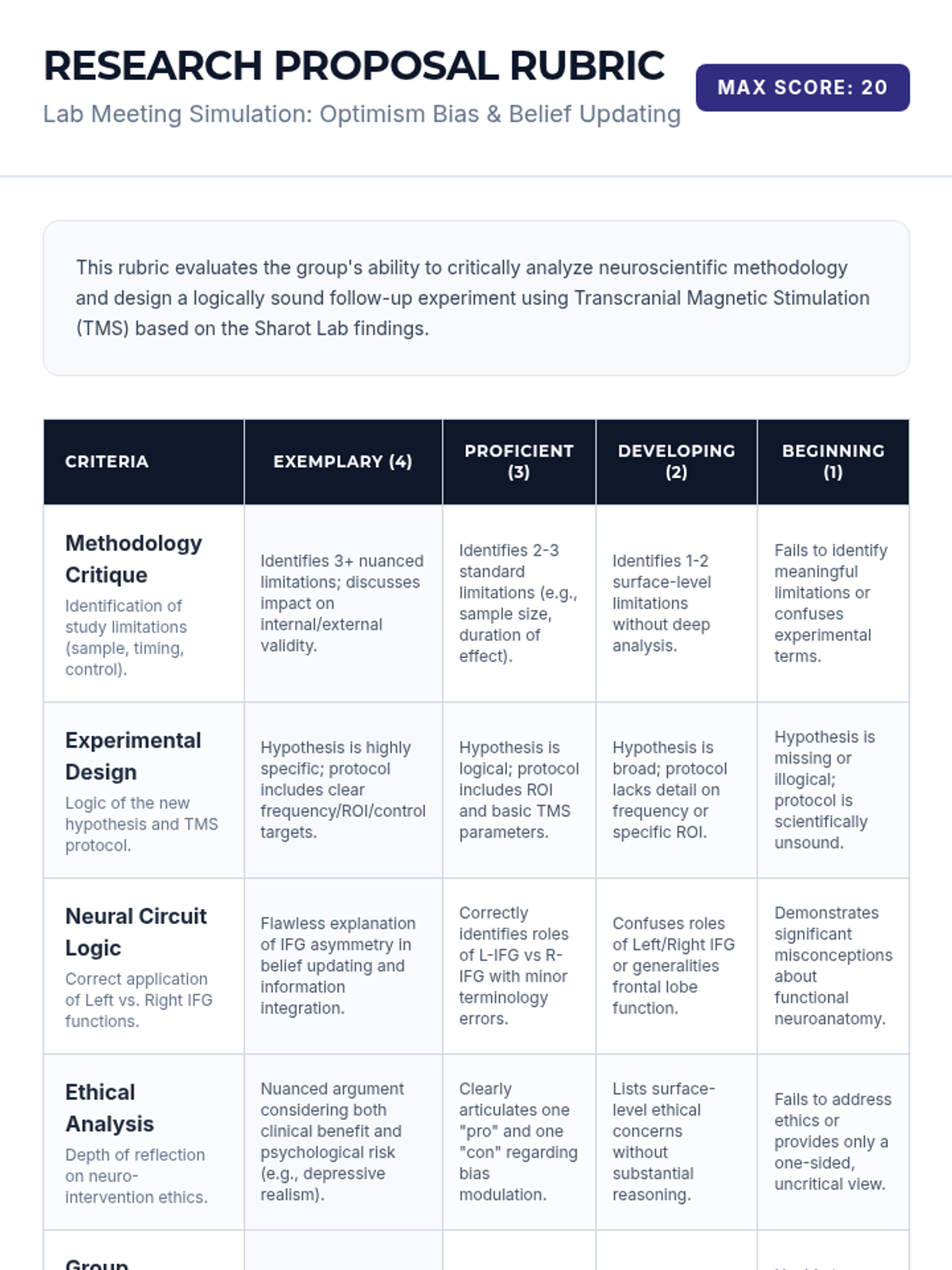
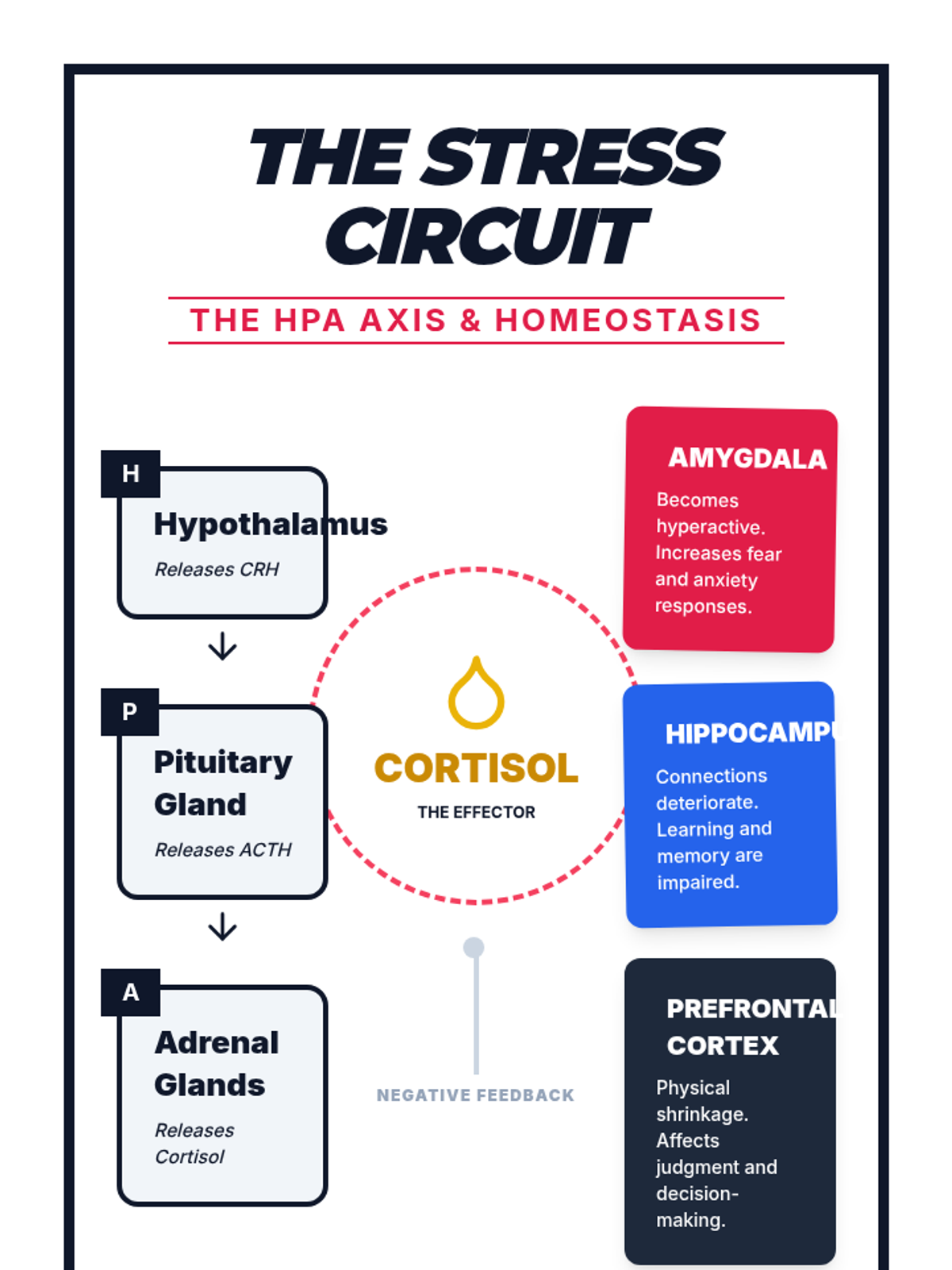
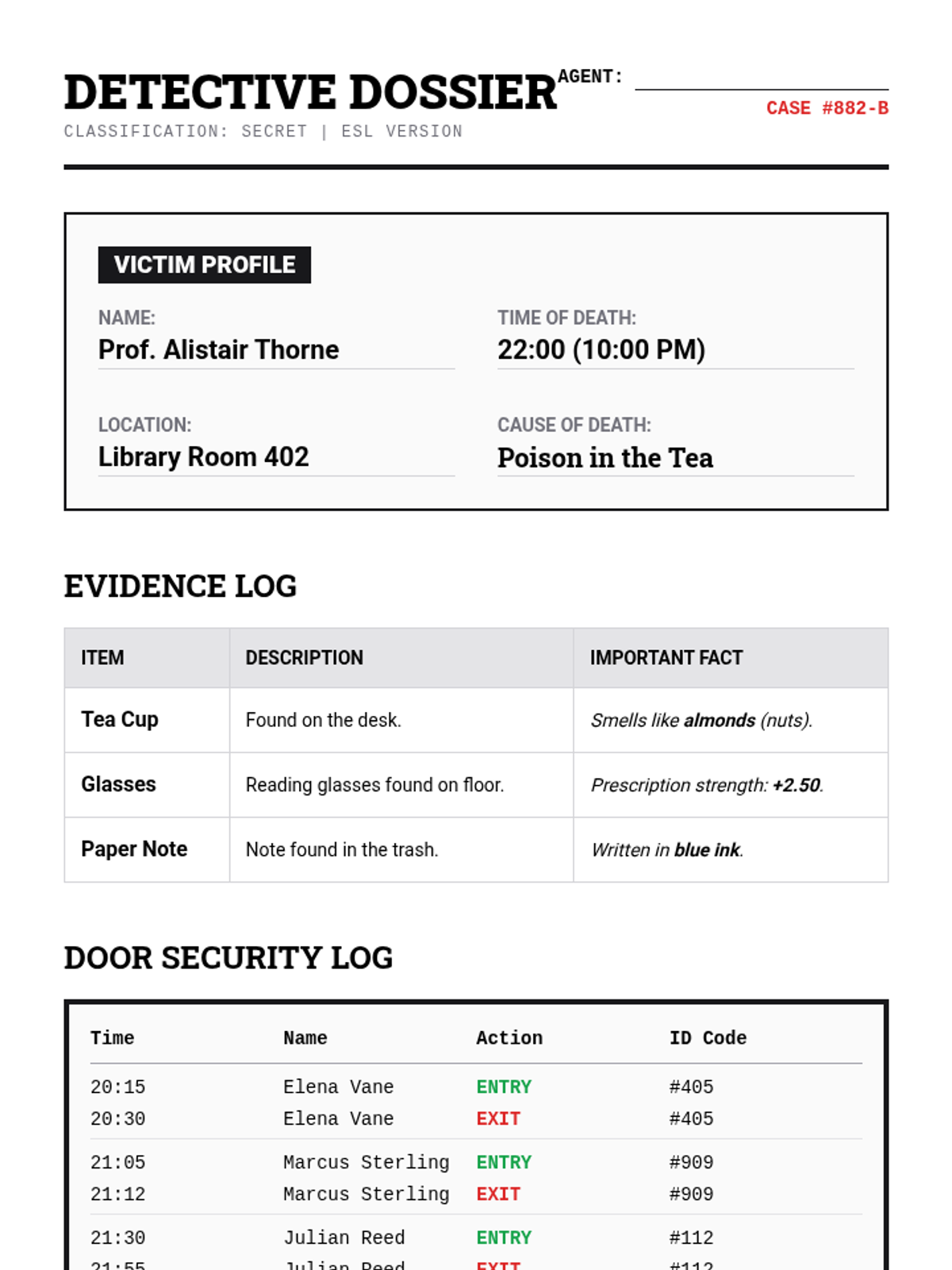
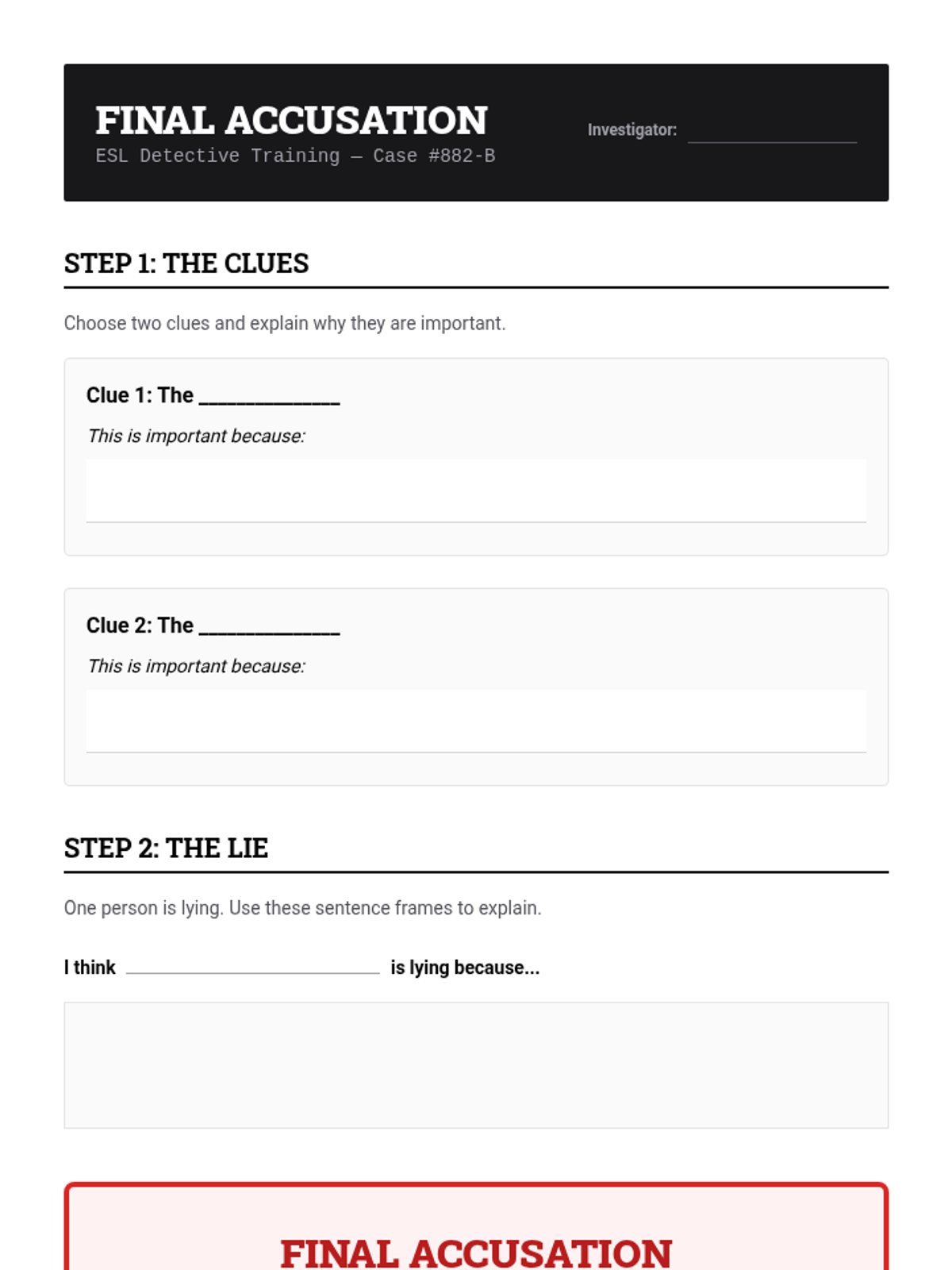
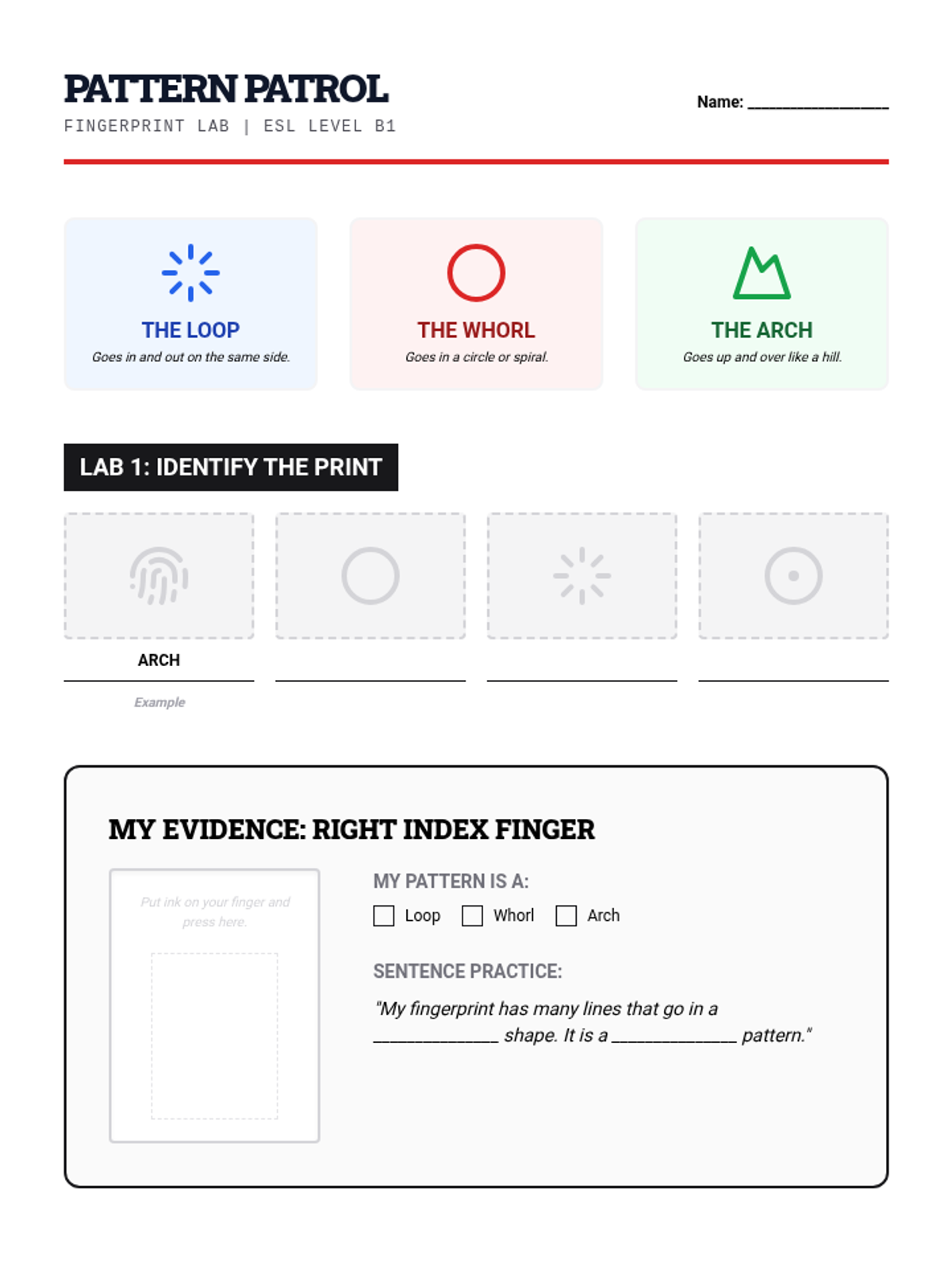
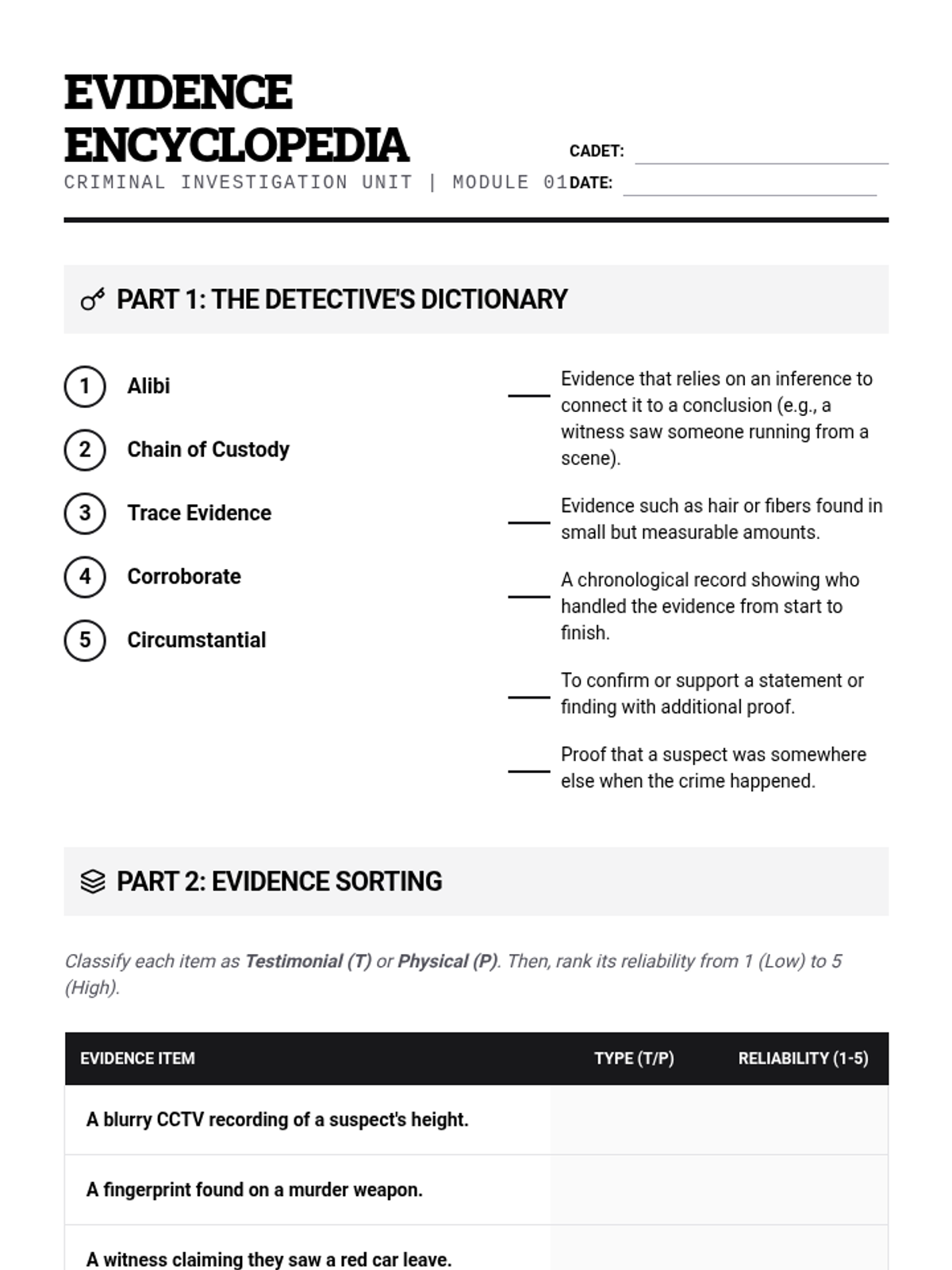
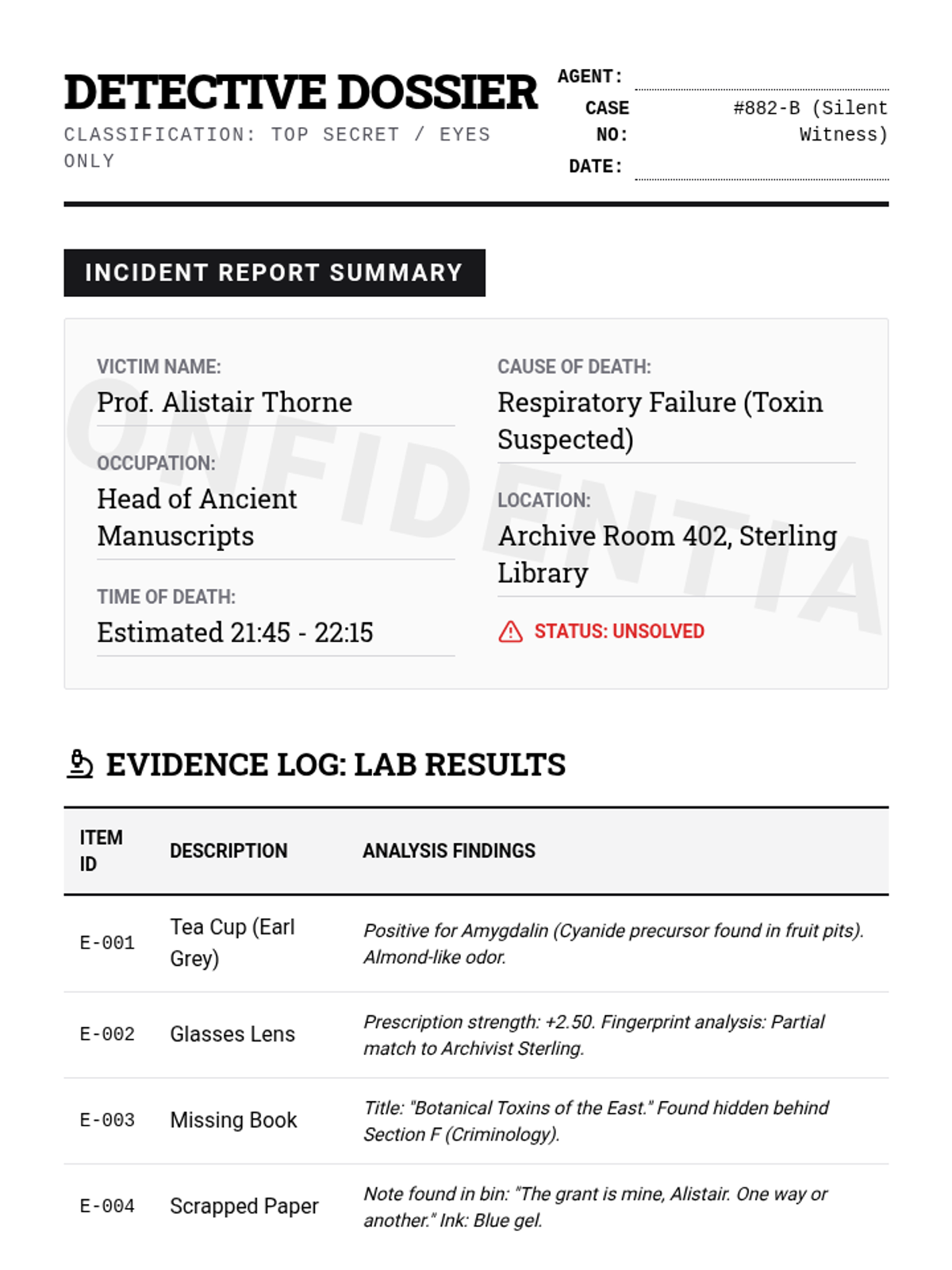
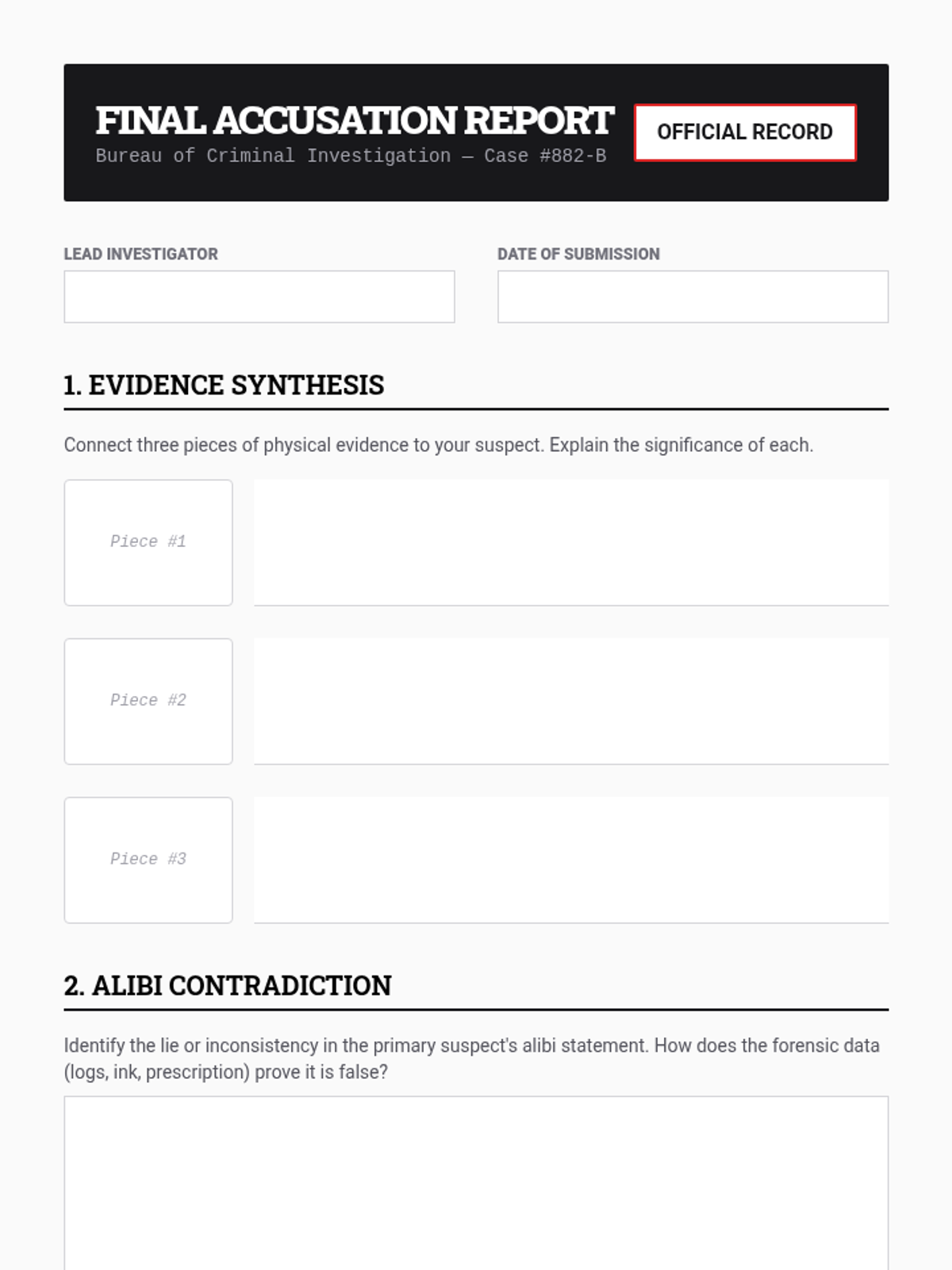
A high school chemistry lesson exploring how quantized energy levels in atoms lead to unique emission spectra. Students compare continuous and discrete light sources, watch a video on quantum principles, and perform a spectroscopy lab using gas discharge tubes.